Abstract
Introduction
Chimeric Antigen Receptor (CAR) T cell therapy is used to treated relapsed/refractory B-Acute Lymphoblastic Leukemia (B-ALL) patients. Long term side effects include B cell aplasia and cytopenia that are treated with supportive therapy. Prolonged cytopenia is seen in 16% of CAR T-cell treated B-ALL patients. The etiology of cytopenia is not clear, and could be attributed to the impact of chemotherapy, CAR T cells and disease or patient-specific factors. The impact of CAR T cell infusion on bone marrow cellularity, lymphocyte compartment and its correlation with cytopenia has not been investigated.
Methods
We analyzed pre- and post-CAR bone marrow biopsies in 178 B-ALL patients who received a CD19-directed CAR T-cell product between 2012-2017 and followed for at least 12 months. Responses were categorized into sustained responders, non-responders, CD19-positive relapses and CD19-negative relapses as previously described. Bone Marrow biopsy (BMB) overall cellularity, complete blood counts (CBC) in a 12-month post infusion period were analyzed. BMB were considered mildly, moderately and severely hypocellular based on cellularity of 50, 25 and 5% respectively. Hypocellularity was further stratified by CBC per aplastic anemia (AA) classification guidelines: Non-Severe Aplastic Anemia (NSAA) and Very Severe Aplastic Anemia (VSAA). Immunohistochemistry (IHC) for CD3, CD4, CD8, CD163, granzyme and perforin were performed on pre- and post-CAR BMB. CD3 IHC was digitally scanned and analyzed quantitatively (Leica Aperio ImageScope) and qualitatively. Targeted RNA sequencing-based gene expression profiling (EdgeSeq Immuno-Oncology Panel, HTG Diagnostics) was performed on pre-and post-treatment biopsies, with differential expression assessed by DESeq2 within HTG Reveal software. RNAseq gene expression was deconvoluted to impute relative expression of immune cell subsets.
Results
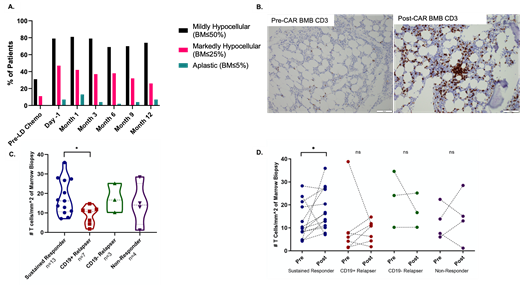
31% of patients were mildly hypocellular but none were severely hypocellular at baseline pre-CAR timepoints. The highest proportion of hypocellularity was at the 1-month time point. 81% of patients were mildly hypocellular (≤50%), 42% were markedly hypocellular (≤25%), and 13% were severely hypocellular (≤5%) at the 1-month time point. By month 12, the proportion of mildly hypocellular patients was 74%, markedly hypocellular patients was 26%, and severely hypocellular patients was 7% (Figure 1). The proportion of NSAA and VSAA was 57% and 10% in moderately hypocellular BMB. The proportion of NSAA and VSAA was 64% and 18% in severely hypocellular BMB. Severely hypocellular patients had a higher average day minus 1 disease burden (33% involvement) compared to their moderate (24%) and non-hypocellular patients (16%). VSAA patients had a lower baseline BMB cellularity (47%) compared to NSAA patients (71%) and those without AA (60%)
Increased CD3+ T cells were noted in the post-CAR BMB compared to the pre-CAR BMB (Figure 1B). Lymphocyte were singly scattered or formed loose aggregates and tight lymphohistiocytic clusters. IHC and RNAseq analysis showed increased CD8+ granzyme+ cells in the post-CAR BMB compared to pre-CAR BMB. Sustained responders showed higher T cell infiltration compared to other categories of patients (Figure 1C and D). Lymphoid infiltrates did not correlate with hypocellularity or cytopenia.
Conclusion
We describe for the first time the changes in bone marrow cellularity and lymphocyte compartment after CD19-directed CAR T-cell infusion in B-ALL. A subset of patients were moderately or severely hypocellular and met criteria for aplastic anemia. However, most of them recovered bone marrow cellularity and CBC. Hypocellularity and AA were correlated with pre-CAR disease burden and baseline cellularity rather than post-CAR lymphocyte infiltration. We also show for the first time that sustained responders to CD19-CAR showed increased CD3+ CD8+ T cell infiltrates compared to CD19-positive relapses and non-responders.
Figure 1. A. Bone marrow hypocellularity post-CAR T-cell infusion. Proportion of patients who were mildly hypocellular, markedly hypocellular, and severely hypocellular in the 12month follow up period. B. Increased CD3+ T cells and aggregates after CAR T cell infusion. C and D. Sustained responders show significantly greater CD3+ T cells after CAR T cell infusion compared to other categories. *P<0.05.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal